 
Mesoionic Compounds -
SYDNONES AND SYDNONE IMINES in AKos
Samples.
Download file with
mesoionic compounds that were synthesized and were once on stock:
mesoionic-stock.sdf,
mesoionic-stock.db (ISIS/Base
file)
Go to a
spreadsheet of ca. 4000 mesoionic compounds or
download the spreadsheet as .xls file. |
| Sydnone
and sydnoneimines are mesoionic aromatic heterocyclics, derivatives of
1, 2,3-oxadiazole system. They possess an unique physical and chemical
properties, which is result of unusual ring construction. Biological properties of
the compounds are of great interest. The title substructure can be
found in the stimulant drugs
feprosidnine and
mesocarb and the
vasodilator
molsidomine. |
| The first review on the
chemistry of the compounds: |
The Chemistry
of Sydnone Imines
V G Yashunskii and L E Kholodov 1980
Russ. Chem. Rev.
49, 28. |
|
Sydnone, the potential basic pharmacodynamic nucleus has been reported |
McNaught A D in, Adv Heterocyclic Chem, Katritzky A R and Boulton A J (Eds.),Advances
Heterocyclic Chemistry, Academic Press New York, 1976, 175-319.
Moustafa M A, Nasr M N, Gineinah M M and Bayoumi W A,
Arch Der Pharmazie,2004,
337,
164-170.
Kavali R Jyoti and Badami B V,
Farmaco,
2000,
55(5),
406-409. |
|
to a wide variety
of biological activities:
Antitumor
(anticancer): |
Dunkley C S and Thoman C J,
Bioorg Med Chem Lett.,
2003,
13(17),
2899-2901.
Grynberg N., Gotes R., Miller J., Echevarria A.// Anticancer.
Res.-1992.-V.12.- N3.- p.1025-1028; C.A. 1992.-117.-142871t.
Dunkley C.S., Thoman C.J. Synthesis and biological evaluation of a novel
phenyl substituted sydnone series as potential antitumor agents.//
Bioorg. Med. Chem. Lett.-13.-2003.- p.2899-2901.
Halila G.C. et.al. Effect of sydnone SYD-1, a mesoionic compound On
energy-linked function of rat liver mitochondria.// Chemico-Biological
interactions.- 169.- 2007.- p. 160-170.
Kier L.B., Roche E.B.// J.Pharm. Sci.- 1965.- V.56.- p.149-167. |
|
Anticancer: |
Thamotharan S, Parthasarathi V, Mallur S, Kamble R, Badami B and Linden
A,
Acta Cryst.,
2004,
E60,
701-702. |
|
Anti-inflammatory
and analgesic: |
Kalluraya B, Rahiman A M,
Polish J Chem.,
1997,
71,
1049-1052.
Hill
J.B., Ray R.E.// J. Med. Chem.- 1975.- V.18.- N1.- p.51-53.
Abdul Rahiman M., Kalluraya B., Hegde J.C. Sydnone derivatives part IX:
synthesis, antimicrobial and pharmacological activity of some
sydnone-N-Mannich bases.// Indian journal of Heterocyclic Chem.-
v.12.-2003.-p.241-244.
Abdul Rahiman M., Kalluraya B. Sydnone derivatives: part X – synthesis
and pharmacological activity of some novel cyclohexenones and
indazolinones.// Indian journal of chemistry – section B Org. and
Med.Chem.- v.42.-2003.-p.1141-1148.
Abdul Rahiman M., Kalluraya B., Banji D. Sydnone derivatives: part V –
synthesis and pharmacological properties of some novel
triazolothiadiazepin.// Indian journal of chemistry – section B Org. and
Med.Chem.-v.41.-2002.-p.1712-1717.
Mackie J.E.// Biochim.Pharmacol.- 1990.- V.39.-N11.- p.1767-1774.
Satyanarayana K., Rao M. N. A.// Indian. Drugs.- V.30.-
N7.-1993.-p.313-318; C.A., 1994.- 120.- 45418e.
Krishna G., Bharathi V.B., Gurubasav S.P.// Arch. Pharm.- 1980.- V.313.-
p.684-688.
Abdul Rahiman M., Kalluraya B., Banji D. Sydnone derivatives. Part VII:
synthesis of some novel triazoles and their pharmacological
properties.// Arch. Pharm. Pharm. Med.Chem.-2001.-334.-p.263-268. |
|
Antiviral : |
Pandey V K and Tandon M,
Indian J Heterocycl Chem,,
2006,
15,
399-400.
Bourinbaiar A S, Tan X and Nagorny R,
Acta Virol.,
1993,
37,
241-250. |
|
Scavenging activity: |
Shih M H and Ke F Y,
Bioorg Med Chem.,
2004,
12,
4633-4633. |
|
Antimalarial: |
Friedman M D, Stotter P L, Porter T H and Folkers K,
J
Med Chem.,
1973,
16(11),
1314-1316. |
| Effective endogenous donor
NO: |
Dendorfer A. // HerZ 1996. D. 21 (Anh. 1). S. 38.
Schonafinger R. // II Farmaco 1999. Vol. 54. P.316.
Newton C.G., Ramsden C. A. // Tetrahedron 1982. Vol. 38. P. 2935. |
|
Aantihypertensive activity, example |
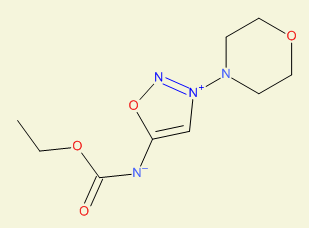
ethoxycarbonyl-(3-morpholinooxadiazol-3-ium-5-yl)azanide
XLFWDASMENKTKL-UHFFFAOYSA-N
InChI=1S/C9H14N4O4/c1-2-16-9(14)10-8-7-13(11-17-8)12-3-5-15-6-4-12/h7H,2-6H2,1H3
Molsidomine |
| Antiischemic
activity |
Bohn H., Deyerle R., Martorana P. A., Schonafinger K.J. //
Cardiovasc. Pharmacol., 1991. Vol. 18. P. 522.
Feelisch M. // Naunyn-Schmiedebergs Arch. Pharmacjl.,
1998. Vol. 358.
Megson I.L. // Drugs Future 2000, 25. P. 701.
Bohn H., Martorana P. A., Schonafinger K.J. // Eur. J.
Pharmacol., 1992. Vol. 220. P. 71. |
| Antiischemic
activity, example |
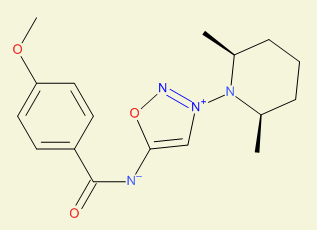
[3-[(2S,6R)-2,6-dimethyl-1-piperidyl]oxadiazol-3-ium-5-yl]-(4-methoxybenzoyl)azanide
InChI=1S/C17H22N4O3/c1-12-5-4-6-13(2)21(12)20-11-16(24-19-20)18-17(22)14-7-9-15(23-3)10-8-14/h7-13H,4-6H2,1-3H3/t12-,13+
TXPUBJSOHAMNEI-BETUJISGSA-N
Pirsidomin |
|
Antithrombotic activity |
Annual Drug Data
Rep. / Ed. J. R. Prous. 1991. Vol. 13, №11. P. 945.
Wang George
Peng, Xian Ming, Tang Xiaoping et. al. Nitric Oxide Donors: Chemical Activities
and Biological Applications. // Chem. Rev. 2002, 102, P. 1091-1134.
Rehse K.,
Schleifer R., Cuborski T. New NO-donors with antithrombotic and vasodilating
activities. II: 3-Alkyl-N-nitroso-5 sydnone imines. // Arch. Pharm. (Weiningem,
Ger.) 1993. V. 326, № 10. P. 791-797.
Rehse K.,
Schleifer K.-J., New NO-donors with antithrombotic and vasodilating activities,
III: 3,4-disubstituted N-nitroso-5 sydnone imines. // Arch. Pharm. (Weiningem,
Ger.) 1993. V. 326, № 12. P. 929-939.
Rehse K., Schleifer K.-J. et. al., New NO-donors
with antithrombotic and vasodilating activities, IV: Chemical Reactivity of
Nitrosimines and its Implications for their Pharmacologic Properties. // Arch.
Pharm. (Weiningem, Ger.) 1994. V. 327, № 6. P. 359-364. |
| Aticancer
[antitumor]: |
Kier L.B., Roche E.B. Molecular orbital calculation of
the electronic structure of the sydnones. // J. Pharm. Sci.
1965.
V.
56,
P.
149-167. |
| Central and peripheral
nervous system: |
Boenringer Ingelheim, Novel Sydnonimine derivatives, the preparation
thereof and compositions containing the same. //
Патент ФРГ
1,198,283, 1967;
С.А.,
1967, 35049.
Pastushenkov B.A., Badyshov B.A. Improvment of human resistanse to high
air temperature using drugs. // (Inst. Aviats. Kosm. Med., Moscow
Russia). Med. Tr. Prom. Ekol. 1995. V. 9. P.
39-42.;
C.A.
1996, 124, 45664.
Li Shuang, Quock Raymond M. Effects of a nitric oxide donor on behavior
and interaction with nitrou oxide in the mouse light/dark exploration
test. // European Journal of Pharmacology, 2002, 447,
Р.
75– 78. |
| Central nervous system: |
Abdul
Rahiman M., Kalluraya B., Banji D. Sydnone derivatives. Part VII:
synthesis of some novel triazoles and their pharmacological
properties.// Arch. Pharm. Pharm. Med.Chem.-2001.-334.-p.263-268.
Kier L.B.// J.Pharm. Sci.- 1967.- V.56.- p.149.
Bheemarao G.U., Bharjti V., Gurubasav S.P.// Arch. Pharm.- 1978.-
V.311.- p.109-114. |
| Fungicidal
activity: |
Pilli H.,
Safak C., Abbasoglu U.// Arch. Pharm (Weinheim, Ger.).- 1993.- V.326.-
N9.- p.559-561; C.A., 1994.- 120.- 244853a.
Lingappa B., Kalluraya B., Nooji S.R. Synthesis and biological study of
some novel 4-[5-(4,6-disubstituted-2-thiomethylpyrimidyl)-41-amino-1,2,4-triazol-31-yl]thioacetyl-3-arylsydnones.//
Phosphorus. Sulfur, and silicon and the related. |
|
Analgetic anticonvulsive spasmolytic: |
Tien Hsien Ju, Tsai Yan Hong, Yeh Wen Yuan; Lee Yaw Kuen,
Ho Yong Shang// J. Chin. Chem. Soc. (Taipei).- 1990.-V.37.- N1.-
p.79-84; C.A., 1990.- 112.- 191419 |
|
Antibacterial: |
UK
1407540 (1975); C.A., 1977.- 87.- 135343f.
Moustafa A. Mohamed et.al. Novel analogues of sydnone: synthesis,
characterization and antibacterial evaluation. // Arch. Pharm. Pharm.
Med.Chem.-2004.-337.-p.427-433.
Moustafa A. Mohamed et.al. Synthesis and biological testing of novel
analogues as potential antibacterial agents. // Arch. Pharm. Pharm.
Med.Chem.-2004.-337.-p.164-170.
Latthe P.R. et.al. Curtius rearrangement reactions of
3-(4-azidocarbonyl)phenylsydnone. Syntesis of 4-(sydnon-3-yl)phenyl
carbamates, N-aryl-N1-[4-(sydnon-3-yl)]phenyl ureas and their
antimicrobial and insecticidal activities.//J.Chem.Sci.-v.118.-N3.-2006.-p.249-256. |
|
Antioxidant: |
Mei-Hsiu
Shih, Fang-Ying Ke. Synthesis and evaluation of antioxidant activity of
sydnonyl substituted thiazolidinone and thiazoline derivatives.// Bioorg.
Med. Chem.-12.-2004.- p.4633-4643.
Mei-Hsiu Shih, Yu-Sheng Su, Cheng-Ling Wu. Synthesis of aromatic
substituted hydrazino-thiazole derivatives to clarify structural
characterization and antioxidant activity between 3-arylsydnonyl and
aryl substituted hydrazino-thiazoles.// Chem.Pharm.Bull.-v.55.-N8.-2007.-p.1126-1135. |
|
 
| |